Research
There is significant variability in health status as people reach old age, ranging from those who are completely independent and robust, to those who have reduced function and poor quality of life. Frailty quantitively captures the heterogeneity of aging, and is a measure of biological, rather than chronological age. Those who are frail are at increased risk of falls, hospitalization and mortality. In fact, frailty is the best predictor of mortality risk. However, little is known about the underlying molecular mechanisms of frailty and whether they are distinct from or overlapping with those of aging. We also have no clinically accepted frailty biomarkers to identify those at risk, or interventions to delay or prevent frailty. Additionally, significant sex differences in frailty are apparent but not well understood. Women are frailer than men at every age but have lower risk of mortality, and the reasons for this are unknown. Through my research I aim to increase our understanding of frailty at the molecular level to transform how we understand diseases and health in aging. Studies in humans are limited by the complexity and heterogeneity of the older population, so I use preclinical frailty tools, reverse-translated from human assessments, which provide ideal models to investigate frailty mechanisms in naturally aging mice.
Development of predictive frailty-based measures
My most recent work has focused on developing the first clocks that model frailty measures in mice to predict outcomes (Schultz*, Kane* et al. 2020). used machine learning algorithms to develop two predictive clocks based on observational non-invasive frailty measures in mice: the FRIGHT (Frailty Inferred Geriatric Health Timeline) clock, a strong predictor of chronological age and the AFRAID (Analysis of Frailty and Death) clock which accurately predicts life expectancy from 21 months of age in male mice. We also showed that these clocks were responsive to healthspan and lifespan interventions including methionine restriction. To encourage use of these clocks by the research community we have built a website with a demonstration video and automated clock-calculators (frailtyclocks.sinclairlab.org). Future work will add molecular and physiological measures to these clocks to further increase their predictive ability and validate these clocks in other mouse strains and female mice.
Can frailty be affected by interventions?
During my PhD, I spent several months in Rafa de Cabo’s lab at the National Institute on Aging, where we completed the first interventional frailty study in mice (Kane, et al. 2016). This important study showed that the known longevity interventions resveratrol, and calorie restriction increased healthspan and delayed frailty in mice. Whilst finishing my PhD I was also involved in the development of the first model of polypharmacy exposure in mice. We found that polypharmacy causes adverse geriatric outcomes in aging mice (Huizer-Pajkos, et al. 2016), and that deprescribing can attenuate this (Mach, et al. 2020). During my first post doc at Dalhousie University I completed the first longitudinal intervention study on the frailty index in mice, exploring the beneficial effect of enalapril on frailty in middle aged and old male and female mice (Keller, et al. 2018). Currently, I am investigating the effect of the NAD+ precursor, nicotinamide mononucleotide (NMN), as an intervention to delay frailty and extend lifespan. NAD+ is an essential co-factor of sirtuins, histone deacytalase proteins that have been associated with increased longevity, and levels of NAD+ decrease by up to 50% in aging. My data suggests that at 23 months of age NMN treated mice have lower frailty scores, lower FRIGHT age scores and higher AFRAID clock scores, indicating that NMN treated mice are less frail, appear younger and are predicted to live longer than controls.
Can we use mouse models to understand sex-differences in frailty?
Sex differences in aging and frailty is a growing area of interest for me, as demonstrated by my recent reviews on sex differences in the aging heart (Kane et al. 2018; Ghimire et al 2017) and sex specific responses to dietary restriction (Kane et al. 2018). During my time at Dalhousie University I completed initial studies exploring sex differences in frailty. We found different rates of frailty development, and differing responses to interventions, in males and females (Kane, et al. 2018; Keller, et al. 2018), with female C57BL/6 mice developing frailty faster than male mice but more responsive to the intervention of enalapril. We measured frailty index in an Alzheimer’s Disease model and found that male 3xTg-AD mice were more frail than females, with parallel increases in mortality (Kane, et al. 2018). We also observed sex specific relationships between frailty and heart aging at the structural and molecular levels with age-related cardiac changes graded by frailty in males, but not females (Kane et al 2020; Kane et al 2020). This suggests that older females may be more resilient and may resist some of the adverse effects of frailty on the heart. This would be consistent with the morbidity-mortality paradox, where older women have higher levels of frailty than men at any age yet live longer, but more research is needed.
What is the link between cardiac aging and frailty?
In humans, being frail increases the risk of cardiovascular disease and vice versa, but the relationship between heart aging and frailty is not well understood. At Dalhousie University, I led work showing that changes to the structure and function of the heart with aging are graded by frailty (Kane, et al. 2020; Feridooni, et al. 2017). Furthermore we showed that frailty grades detrimental changes in the heart at the molecular level, at least in male mice (Kane, et al. 2020). These observations suggest that differences in overall health status contribute importantly to the impact of age on the heart, and that underlying mechanisms including inflammation may contribute to both detrimental changes to the heart with aging and the development of frailty.
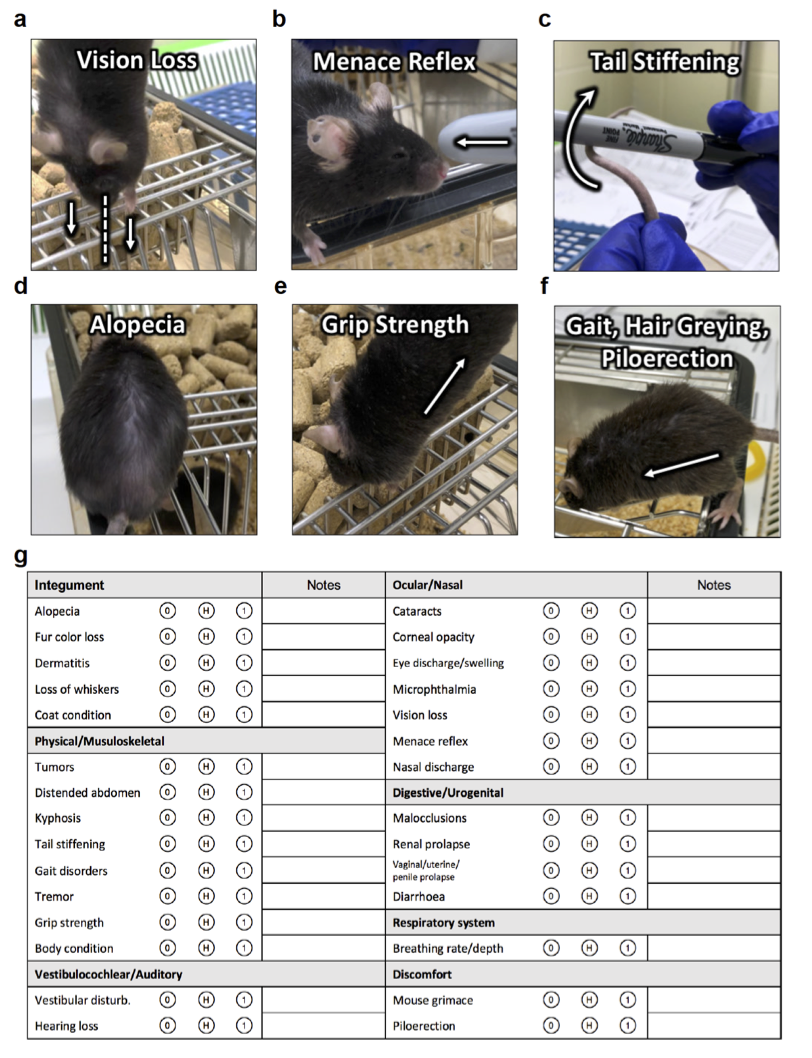
Development and optimization of frailty-based assessments for aging rodents.
The recent development of tools to assess frailty in naturally aging preclinical models, has created an exciting and growing area of research within the aging field. My experience and work in this area has allowed me to position myself as one of the experts in the growing area of preclinical models of frailty. I co-wrote a guest editorial on advances in this field for a special issue of the Journals of Gerontology: Biological Sciences (Kane, et al. 2017), and co-guest edited a special Biology of Frailty issue for Mechanisms of Aging and Development in 2019. During my postdoc at Dalhousie University I collaborated on a project to develop the first frailty index assessment tool for use in aging rats, an important contribution to the field (Yorke, et al. 2017). A study directly comparing the two main tools for frailty assessment in mice that I completed during this time highlighted important similarities and differences between these tools (Kane, et al. 2016). I developed a frailty assessment based on deficits in laboratory measurements and found important sex differences in the association between frailty and inflammation in male and female mice (Kane, et al. 2018). I have also written several reviews and chapters on animal models of frailty, and their applications for studying frailty mechanisms and interventions (Kane, et al. 2016; Kane et al. 2017; Kane et al 2019a; Kane et al 2019b).
How does acetaminophen hepatotoxicity risk change with age and frailty?
Acetaminophen is a commonly used analgesic, especially in the older population, that can cause hepatoxicity in overdose situations. Despite the high prevalence of drug use, pain and adverse drug reactions in the older population, there are limited studies on the effect of aging on acetaminophen toxicity. During my undergraduate research year I contributed to the first review on acetaminophen toxicity in old age (Mitchell, et al. 2011). In this same year I completed a retrospective clinical study investigating the characteristics of acetaminophen overdoses in older and younger patients, which found that older patients were more likely to have chronic, accidental overdoses, rather than acute intentional overdoses (Kane, et al. 2012). My subsequent PhD studies focused on understanding the changing risk to acetaminophen toxicity with increasing age in aging mouse models. I found that acetaminophen toxicity risk was not increased in aging or frail mice in both acute and chronic exposure models (Kane, et al. 2016). Importantly, however, we discovered that the therapy to treat acetaminophen toxicity, N-Acetyl Cysteine, did not protect against toxicity in chronic exposure models, which has important implications as these are the most common exposures in the older population (Kane, et al. 2016). During my graduate studies I also contributed to studies exploring the effect of old age on isoniazid toxicity, and the associated pharmacokinetic and apoptosis-related mechanisms (Mach, et al. 2015; Mach, et al. 2016).